Simple, rapid AAV characterization
The Refeyn SamuxMP is a mass photometer optimized for adeno-associated virus (AAV) characterization and is an valuable analytical tool for laboratories working with AAVs. SamuxMP mass photometry measurements are quick and require very little sample.
The SamuxMP accurately measures the empty/full capsid ratio for AAVs of any serotype as well as the quantities of partially filled and overfilled capsids. It can also detect impurities and aggregation, and give a rough capsid titer estimate as part of the standard data analysis procedure.
Key Features
1. Quantitative characterization of AAVs of all serotypes
2. Easy measurement of empty/full ratio of AAV capsids
3. Rapid analysis requiring minimal sample
4. User-friendly operation
Transformative AAV analytics
Mass photometry is a bioanalytical technology that measures the mass of individual AAV particles in solution, quickly and using small amounts of sample.
Mass photometry is ideally suited for measurements of empty/full capsid ratios. Filled AAV capsids have greater mass than empty capsids, so they can be readily distinguished from empty capsids on mass photometry histograms (Fig 1).
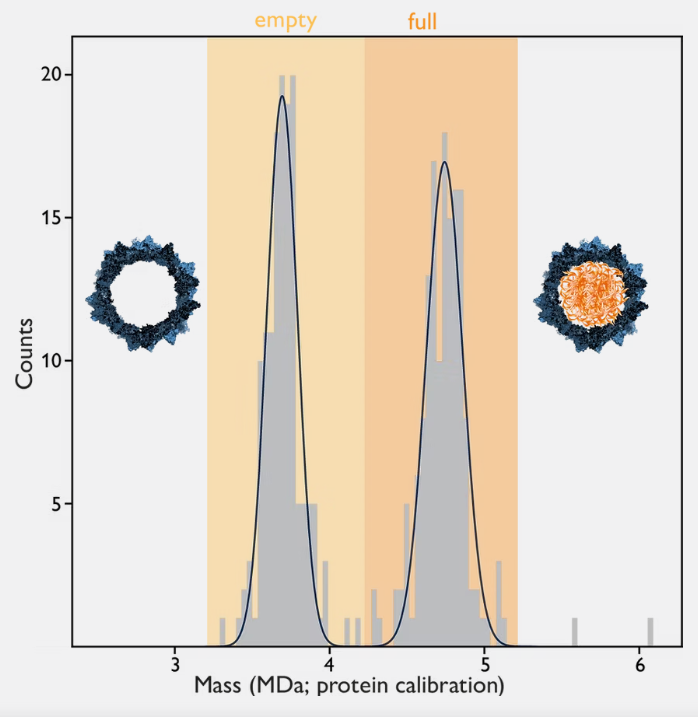
Figure 1 Mass photometry measurement of AAV capsids (AAVDJ serotype) with SamuxMP. Two distinct peaks are visible: one with lower mass corresponding to empty capsids, and a second with higher mass corresponding to full capsids.
Serotype-agnostic AAV analytics
The SamuxMP mass photometer can measure AAV capsids of any serotype without the need for protocol adjustments.
Figure 2 shows SamuxMP measurements of empty AAV capsids of four different AAV serotypes.
For each sample, the mass photometry measurements with the SamuxMP revealed a single, symmetric peak at the expected mass, confirming that the SamuxMP produces consistent results across serotypes.
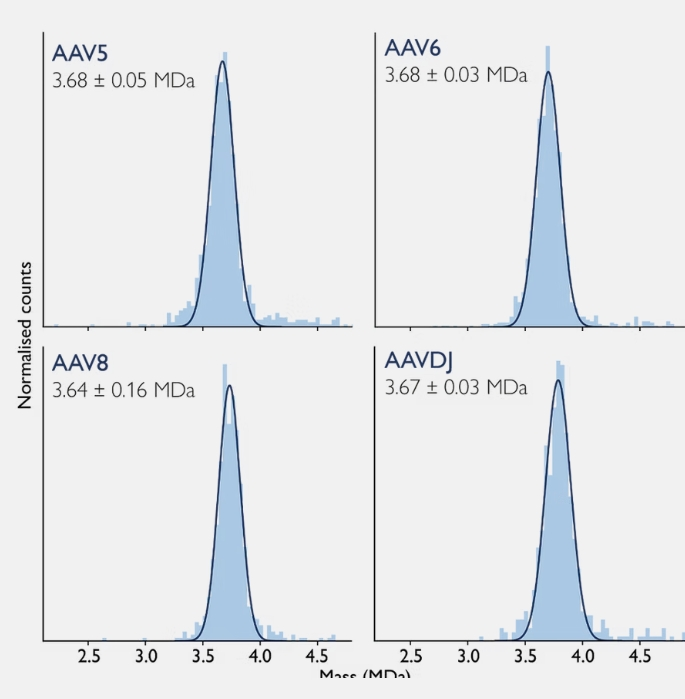
Figure 2 Mass photometry measurements of empty AAV capsids of different serotypes on the SamuxMP. Mass histograms for four different AAV serotypes, with the mass and standard deviation calculated from three technical replicates.
SamuxMP software package for GMP
This software solution expands the use of the SamuxMP and the SamuxMP Auto mass photometers to GMP – regulated environments and enables their use within AAV-based gene therapy manufacturing.
The software package supports compliance with the FDA 21 CFR 11 (US) and EU GMP Annex 11 regulations. It includes three applications: ManageMP, AcquireMP and EvaluateMPS.
The Refeyn service teams also provide installation and operational qualifications (IQ/OQ), as well as documentation and on-site training.
(Please refer to the product mannual for information details.)




